-40%
Fluke index 2 Sp02 / Spo2 / Oximeter simulator / tester / analyzer /version 2.11
$ 316.8
- Description
- Size Guide
Description
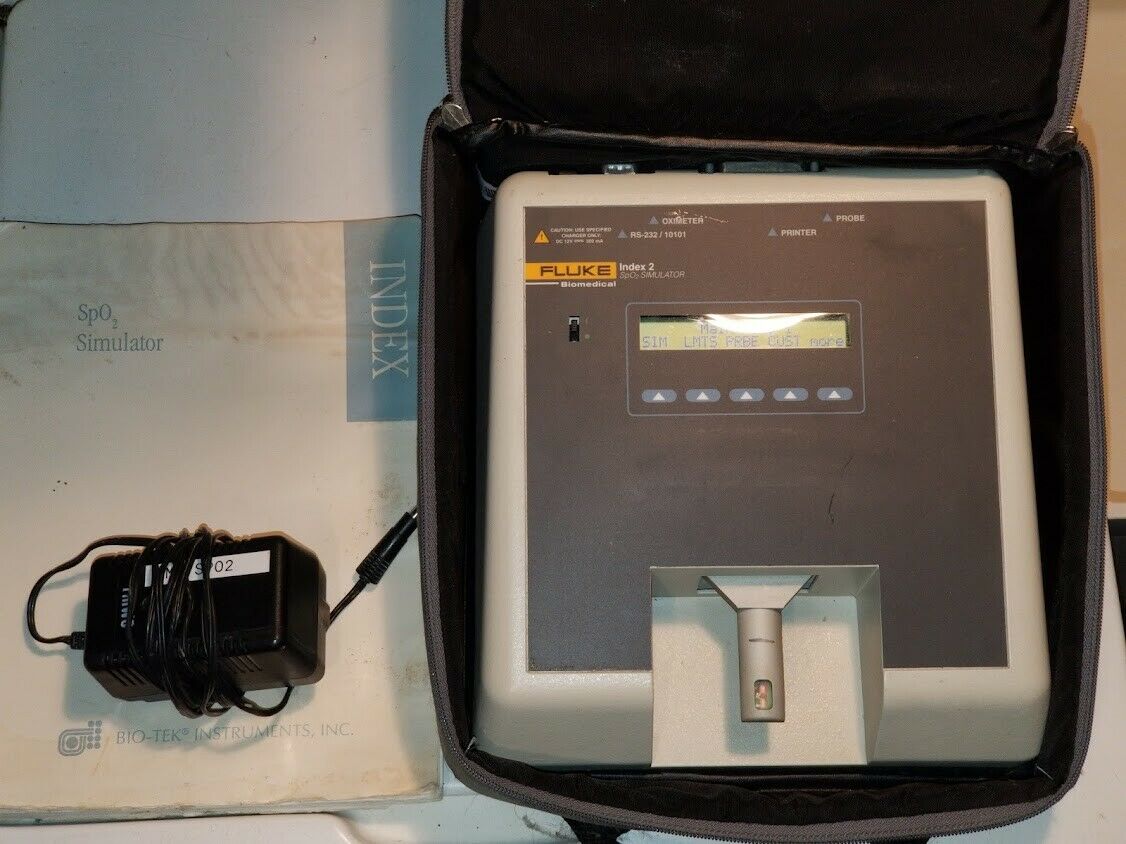
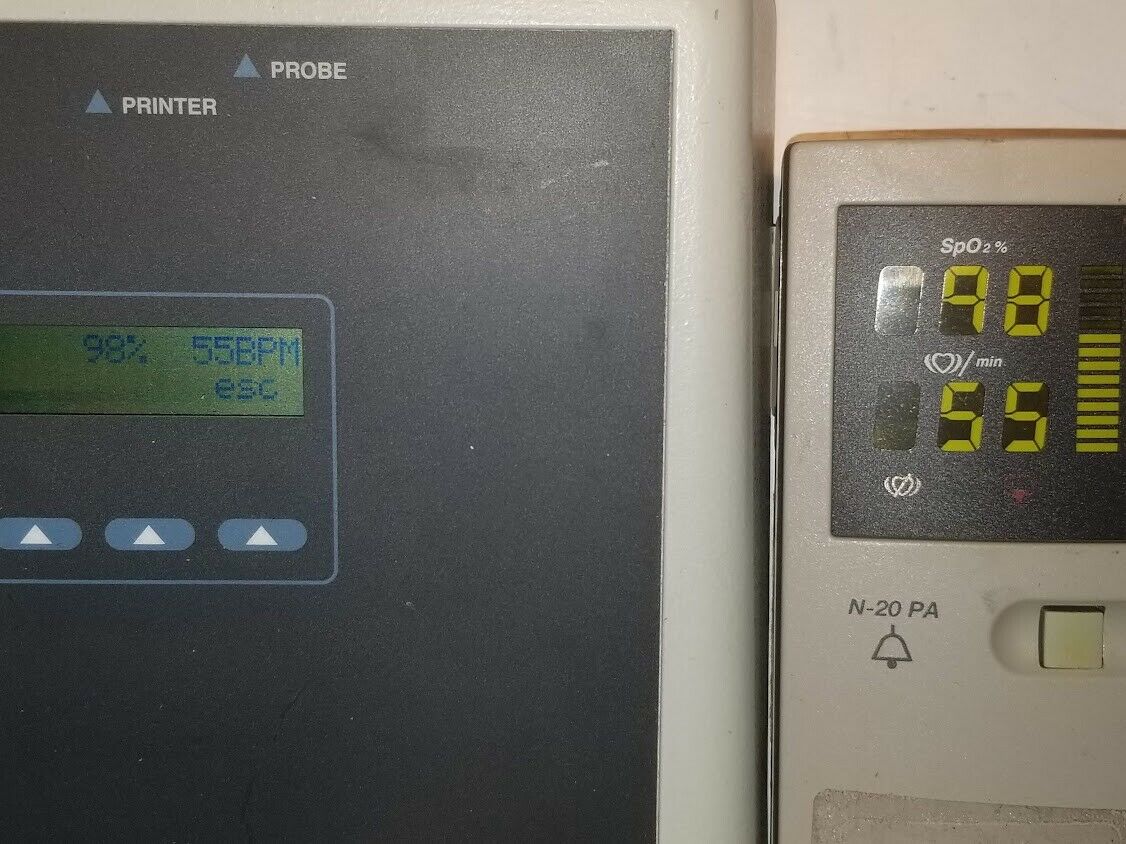
Offered for sale is a Fluke SPo2 simulator with case, power supply, and manual as shown in the picture. The internal battery is dated Jan. 2021. NOTE- the oximeter pictured is NOT included in this sale, as it is for our testing purposes. This has been bio-med checked, and may be returned within 30 days for a full refund.Shipping within the U.S. will be a flat rate . International bidders contact us for a shipping quote. Please E-mail with any questions.
DUE TO RECENT NEGATIVE EXPERIENCES WITH ONLINE BIDDERS, WE ARE ADDING THE FOLLOWING DISCLAIMERS AND CONDITIONS TO OUR LISTINGS:
DUE TO THE HIGHLY TECHNICAL NATURE OF OUR BUSINESS, WE ASK YOU READ OUR LISTINGS COMPLETELY AND UNDERSTAND THE USE AND TECHNICAL / CLINICAL APPLICATIONS OF WHAT YOU ARE BUYING.
PLEASE CONTACT US WITH QUESTIONS
BEFORE
BIDDING..WE ARE VERY HAPPY TO HELP.
WE MAY NOW CHARGE A 20% RESTOCKING FEE ON RETURNED ITEMS THAT ARE NOT DEFECTIVE.
WE WILL NOT BE HELD LIABLE FOR ANY DAMAGE CAUSED BY INTERNATIONAL CUSTOMS INSPECTIONS, AND YOU ARE RESPONSIBLE FOR ANY DAMAGE CAUSED BY YOUR CUSTOMS DEPARTMENT ON RETURNED ITEMS.
OUTSIDE CONDITION OF THIS ITEM IS GOOD.
BY PURCHASING THIS UNIT YOU AGREE TO THE FOLLOWING:
BUYER IS RESPOSIBLE FOR HAVING UNIT INSPECTED BY A
LICENSED TECH BEFORE USING UNIT ON ANY PERSONS,
MINNESOTA MEDICAL RESELLERS WILL NOT BE HELD LIABLE
FOR ANY POSSIBLE MALFUNCTION OF THE UNIT OR MISUSE OF
THIS UNIT OR HARM TO ANY PERSONS BY THIS UNIT.
"The sale of this item may be subject to regulation by
the U.S. Food and Drug Administration and state and
local regulatory agencies. If so, do not bid on this
item unless you are an authorized purchaser. If the item
is subject to FDA regulation, I will verify your status
as an authorized purchaser of this item before shipping
of the item.
If you have questions about legal obligations regarding
sales of medical devices, you should consult with the
FDA's Center for Devices and Radiological Health:
Links:
http://www.fda.gov/cdrh/devadvice/
or
http://www.fda.gov/cdrh/industry/support/index.html
Phone number: 800.638.2041 email:
[email protected]